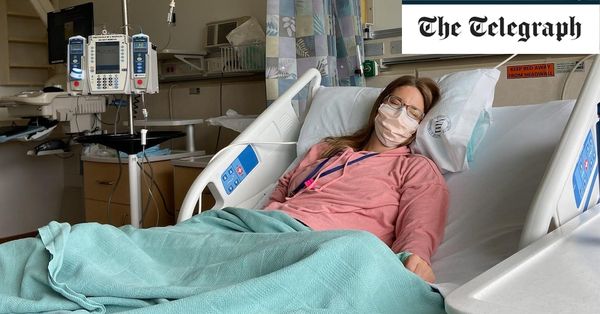
An American woman, Brianne Dressen, has taken legal action against AstraZeneca, claiming that the company’s Covid vaccine has left her permanently disabled. Ms. Dressen participated in a vaccine trial in the US in 2020 and developed a severe neurological condition afterward.

She alleges that AstraZeneca failed to provide medical care for her side effects, leading to her current state. Ms. Dressen’s lawsuit is the first of its kind in the US, where the AstraZeneca vaccine was tested but never approved for use.
The British-made vaccine is currently facing legal action in the UK as well, with over 50 people already filing a class action lawsuit against AstraZeneca. This could potentially result in a multimillion-pound payout. Additionally, the company recently requested the EU to withdraw authorization for its vaccine in member states.
Ms. Dressen claims that she signed an agreement with AstraZeneca, stating that the company would cover the costs of medical treatment for any research-related injuries, provided that the costs were reasonable and not self-inflicted. However, when she experienced severe pins and needles sensations after receiving the vaccine, AstraZeneca did not support her medical care expenses.
Ms. Dressen has been diagnosed with peripheral neuropathy, a condition that causes numbness and pain due to damaged nerves. She is unable to work and has described her condition as “post-vaccine neuropathy” because of its connection to the jab. Hospitalized multiple times after her vaccination, Ms. Dressen has accumulated thousands of dollars in medical bills. She refused a small payout from the company that would have limited their liability in any potential lawsuit.
The impact of Ms. Dressen’s illness has been especially hard on her two children, who are now aged nine and eleven. She stated that they don’t remember her as she was before her condition, and this has been the most difficult part for her. Ms. Dressen volunteered for the clinical trial with the intention of supporting her community, but she feels that she was treated as “nothing more than a number” by AstraZeneca.
Utah law allows complainants who sue for breach of contract to claim costs resulting from the breach and seek damages. Ms. Dressen hopes for a significant payout if the court rules in her favor. Her legal expenses have already reached tens of thousands of dollars, and she is also claiming additional damages for emotional distress, lost income, transportation, and legal fees.
Although the AstraZeneca vaccine has been credited with saving six million lives during the pandemic, it has also been associated with dozens of deaths in the UK due to blood clots. The company recently acknowledged, for the first time, that the vaccine could cause blood clots in very rare cases. In the US, the AstraZeneca vaccine trial involved 32,000 participants and concluded that it was 79% effective against Covid-19. Despite the US government purchasing millions of doses of the vaccine, it was never authorized for public use.
AstraZeneca declined to comment on the ongoing litigation, reiterating that patient safety is their highest priority. They emphasize that clinical trial evidence and real-world data consistently show an acceptable safety profile for their vaccine, with the benefits of vaccination outweighing the risks of extremely rare potential side effects. The company is proud of the role their vaccine has played in ending the global pandemic, as it has been recognized and supported by governments worldwide.